Abstract
Introduction: Immune checkpoint blockade with anti PD-1 antibodies in Hodgkin Lymphoma has been transformative, changing the standard of care in the relapsed/refractory settings. Limited prospective data exist to support a role for anti PD-1 monotherapy for patients (pts) with diffuse large B cell lymphoma (DLBCL) and T cell lymphomas (ORR 36% DLBCL, n=11 ORR 17% T cell lymphomas, Lesokhin et al, JCO 2016). However, in clinical practice, agents such as pembrolizumab and nivolumab are sometimes administered to non-Hodgkin lymphoma (NHL) pts who are without other conventional treatment options. We performed an analysis of all NHL pts treated with anti PD-1 antibodies at our institution to assess activity and safety of anti PD-1 antibodies in the management of relapsed/refractory NHL.
Patients and Methods: We conducted a retrospective cohort study of all adult pts receiving pembrolizumab or nivolumab for management of NHL at the University of Pennsylvania between 1/2014 and 6/2017. Demographics, duration of therapy, reason for discontinuation, overall response rates, survival, and toxicity were examined. The primary endpoint was progression-free survival (PFS) defined as the time of immune checkpoint blockade start to progression, death, or last-follow-up via Kaplan Meier (KM) method. All other analyses were descriptive.
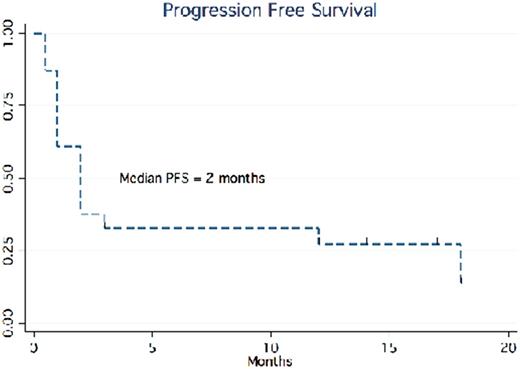
Results: We identified 23 NHL pts for this analysis. NHL subtypes included DLBCL (86%) and peripheral T-cell lymphomas (14%). Median age at time of initiation of PD-1 inhibitor was 65 years old (Range: 21-79). Pts had an Ann Arbor Stage 2 or greater (78%) and median ECOG performance status of 1 (Range: 0-3). High risk features, defined as C-myc rearrangement, Ki-67>80%, high IPI, or ABC subtype, were observed in 15 pts (65%). Majority of pts had no history of autoimmune disease (91%). Pts had a median of 5 prior therapies (Range: 1-14, 91% ≥ 3 prior therapies) including 7 pts who had a prior autologous stem cell transplant (30%). Time to initiating PD-1 inhibitor from last active therapy was 2 months (Ranging from 0.5 to 15 months). In our cohort, 14 pts (61%) received pembrolizumab and 9 pts (39%) received nivolumab. The majority (%) of both groups were administered weight based dosing given in standard frequencies according to each respective package label. Median time on immunotherapy was 1 month ranging from 0.5 to 19 months. Pts most commonly discontinued PD-1 therapy for progression or lymphoma associated mortality (73.4%), and 5 pts (21.7%) remain on active therapy. Overall response rate (ORR) for the entire cohort was 23% (9% CR) and ORR in DLBCL of 26% (N=19). Median PFS for the entire cohort and DLBCL subset was 2 months (median follow-up of 2 months, figure 1). Of the responders (n=5), the median duration of response was 17 months (median of 6 prior therapies).
No pt experienced opportunistic infections while on anti PD-1 therapy. Roughly 47.8% experienced at least 1 toxicity. There were 3 pts (13%) who were documented with at least two concomitant adverse events (AEs). Observed AEs included: pneumonitis (30%), elevated liver function tests (LFT's) (7%), diarrhea/colitis (7%), infusion related reactions (7%), nausea (7%), sepsis (7%), tumor flare (7%), graft versus host disease (GVHD) exacerbation (7%), headache (7%), idiopathic thrombocytopenic purpura (ITP) (7%), and rash (7%). Only 2 (14%) pts required dose interruptions and 57% of AEs resolved, with standard management of immune mediated AEs.
Conclusion: Our results are consistent with the published literature in that we identified a subset of heavily pre-treated multi-refractory NHL pts who achieved durable responses to anti PD-1 antibody monotherapy. While the median PFS / OS for the entire cohort is 2 months, responders, with 6 median prior therapies, achieved a median PFS of 17 months. These results underscore the importance of immune check point blockade in some lymphomas and the critical need to identify biomarkers of response to anti PD-1 therapies so that this therapeutic approach can be delivered to the appropriate pts. A multicenter analysis with correlative studies is in progress.
Svoboda: Kite: Consultancy; Seattle Genetics: Consultancy, Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; BMS: Consultancy, Research Funding; Celgene: Research Funding. Schuster: Bristol-Myers Squibb: Consultancy, Research Funding; Merck: Research Funding; Novartis: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Research Funding; Nordic Nanovector: Consultancy; Seattle Genetics: Consultancy. Landsburg: Curis: Consultancy, Research Funding; Takeda: Research Funding. Dwivedy Nasta: Incyte: Research Funding; Takeda: Research Funding; Immunogen: Research Funding. Mato: TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; DTRM: Research Funding; Portola: Research Funding; Janssen: Consultancy; AstraZeneca: Consultancy; Regeneron: Research Funding; Pharmacyclics: Research Funding; Acerta: Research Funding; Kite: Consultancy; AbbVie: Consultancy, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc.: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.